Histone deacetylase inhibitors
Histone deacetylases (HDACs) are enzymes that catalyze the deacetylation of lysine residues located in the NH2 terminal tails of core histones, which is associated with transcriptional silencing. There are 18 known human histone deacetylases, grouped into four classes based on the structure of their accessory domains. Class I (HDACs 1-3 and 8), II (HDACs 4-7, 9, and 10), and IV (HDAC 11) enzymes are Zn2+-dependent enzymes and are called HDACs, while class III enzymes (also known as sirtuins) are defined by their dependency on NAD+.
Histone deacetylase inhibitors (HDACis) are emerging as a new class of anticancer drugs and have been shown to alter gene transcription and exert antitumor effects such as growth arrest, differentiation, apoptosis, and inhibition of tumor angiogenesis.
The interest in HDAC inhibitors began almost 30 years ago during some studies designed to understand why DMSO caused terminal differentiation of murine erythroleukemia cells. This early observation paved the way for the development of novel pharmacological agents in the field of chromatin remodeling. In October 2006 the FDA approved the first HDAC inhibitor – Suberoylanilide Hydroxamic Acid (SAHA, Zolinza, Vorinostat) to treat the rare cancer cutaneous T-cell lymphoma (CTCL).
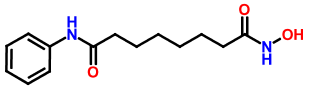
One of the most well-known HDAC inhibitors is a drug called suberoylanilide hydroxamic acid (SAHA)
Based on their chemical structure, histone deacetylase inhibitors inhibitors can be subdivided into four different classes, including hydroxamates, cyclic peptides, aliphatic acids and benzamides. TSA, a compound of hydroxamates, is the first nature product that has been discovered to possess the HDAC inhibitor activity in 1990. Other compounds, for example, CBHA and Panobinostat, have been used in pre- and clinical trials in this group. Another class of HDAC inhibitors is aliphatic acid, including Valproic acid (VPA), phenylbutyrate. The third group is benzamide consisted of Entinostat and MGCD0103. The last group is cyclic peptide including FK-228.
The following HDAC Inhibitors are currently undergoing clinical trials:
Tacedinaline (CI-994, PD-123654, GOE-5549, Acetyldinaline); Entinostat (SNDX-275, MS-27-275, MS-275); BML-210; M344; Dacinostat (LAQ824,NVP-LAQ824); Panobinostat (LBN-589, LBH589, Panobinostat, LBH-589, NVP-LBH589); Mocetinostat (MGCD-0103); Belinostat (PX105684, PXD101); CBHA; PCI-24781 (CRA-024781); ITF2357; Trichostatin A; Apicidin; CUDC-101; Droxinostat; JNJ-26481585; MC1568; SB939; Tubastatin A.
HDAC inhibitors are promising new targeted anti-cancer agents. These substances cause cancer cell growth arrest, differentiation, apoptosis and cell death of a broad spectrum of malignant cells, both solid tumors and hematologic malignancies. Normal cells are much less sensitive to HDAC inhibitors than transformed cells.
References:
1) Jiahuai Tan, et al. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. Journal of Hematology & Oncology 2010, 3:5
2) Philip Jones, et al. A Novel Series of Potent and Selective Ketone Histone Deacetylase Inhibitors with Antitumor Activity in Vivo. Journal of Medicinal Chemistry, 2008, 51, 2350–2353.
3) Nancy Zhou, et al. Discovery of N-(2-Aminophenyl)-4-[(4-pyridin-3-ylpyrimidin 2-ylamino)methyl]benzamide (MGCD0103), an Orally Active Histone Deacetylase Inhibitor. Journal of Medicinal Chemistry, 2008, 51, 4072–4075
4) Marielle Paris, et al. Histone Deacetylase Inhibitors: From Bench to Clinic. Journal of Medicinal Chemistry, 2008, 51(6), 1505-29.
StumbleUpon | Digg | Del.icio.us | Newsvine | Spurl | Reddit




